Monophasic and biphasic dosage are types of liquid dosage forms used for oral, external, special, and parenteral administration. However, there are some advantages, applications, and differences between monophasic and biphasic liquid dosage forms, so let's take a look at them.
Medication is more effective when they reach their site of action hence different dosage forms and routes are used to treat the various diseases. The dosage form is the medium that delivered medication to their sites of action within the body. It is a pharmaceutical product that contains a combination of drugs and excipients, formulated in a particular configuration and apportioned into a specific dose. Pharmaceutical dosage forms are classified as solid dosage form, semi-solid dosage form, liquid dosage form, and gaseous dosage form based on their physical form.
Liquid dosage forms are pourable pharmaceutical formulations that contain a mixture of active pharmaceutical ingredients (API) and excipients dissolved or suspended in an appropriate solvent or mixture of solvents. It is intended to provide a maximum therapeutic effect to patients who have trouble swallowing oral solid dosage forms.
Monophasic liquid dosage forms:
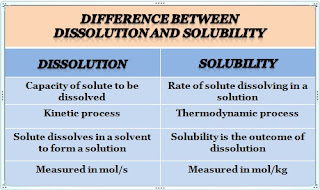
The monophasic dosage form is a single-phase system consisting of two or more components. It is the simple form of presenting the medication for rapid and high absorption of the drug. This is represented by the true solution and the true solution is a clear homogeneous mixture prepared by dissolving the solute in an appropriate solvent. The component of a solution that is present in large quantities is called "SOLVENT," while the component that is present in small quantities is called "SOLUTE."
Classification of monophasic liquid dosage forms:
Monophasic liquid dosage forms for oral use: example- linctuses, mixtures, syrups, elixirs, drops, and draughts, etc.
Monophasic liquid dosage forms for a particular use: example- nasal drops, ear drops, eye drops, eye lotions, mouthwashes, aerosols, gargles, inhalations, and throat paints, etc.
Monophasic liquid dosage forms for external use: example-lotions and liniments etc.
Parenteral solutions: example- intravenous, subcutaneous, and intramuscular injections, etc.
Advantages of monophasic liquid dosage forms:
- It rapidly absorbs the drug than solid dosage form since the drug is already in the form of a solution.
- Because it is homogeneous, it provides a more consistent dose than suspension or emulsion, which requires shaking.
- It can be administered by various routes such as oral, parenteral, otic, ophthalmic, and nasal.
- As compared with solid dosage forms (tablets, capsules) it is easy to swallow, especially for children and old patients.
- The unpleasant taste and smell of the drug are masked by adding sweeteners and flavor-enhancing agents.
Disadvantages of monophasic liquid dosage forms:
- It may undergo hydrolysis if exposed to direct sunlight, so it needs to be store in a cool and dark place.
- The stability of the drug is reduced by hydrolysis or oxidation.
- Water is used in the formulation as a vehicle that is susceptible to microscopic growth; as a result, a preservative is required.
Biphasic liquid dosage forms:
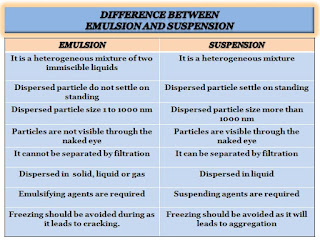
It is a liquid dosage form is one that has two phases; suspensions and emulsions are two examples of biphasic liquid dosage forms. A biphasic system consists of a solid dispersed state and a liquid continuous phase that can be aqueous or oily and is stabilized by the addition of a suspended agent. These are commonly taken orally or through the parenteral route, and they are also used for external applications.
It is used when the drugs are poorly soluble in the solvent. Drugs can be either liquid or solid from the dispersed phase of the system, when the solid drug is distributed in the dispersion medium; the formed system is called a suspension. When the liquid drug is delivered into the dispersion medium, the system form is called emulsion.
Classification of biphasic liquid dosage forms:
The biphasic liquid dosage forms have two types, emulsion and suspension.
Emulsion dosage form: It is a thermodynamically unstable system consisting of two immiscible liquid phases, one of which is dispersed as globules within the other and stabilized by an emulsifying agent. It has two types, water in oil type (w/o), and oil in a water type (o/w). Examples-application, liniment, and lotion, etc.
Suspension dosage form: It is a biphasic liquid or semi-solid dosage form, in which insoluble solid particles of the drug are homogeneously dispersed in a liquid or semi-solid medium. Examples- Gels, lotion, aerosols, inhalation, and eye drop, etc.
Advantages of biphasic liquid dosage forms:
- It is used for the drugs that are insoluble in solution; therefore it is necessary to prepare an insoluble form of the drug, which is then administered as a suspension.
- It exhibits higher bioavailability rates than other dosage forms.
- Suspension can offer prolonged action of the drug since solid particles must be dissolved before absorption.
- Two incompatible ingredients/drugs can be included in a dose, one in each phase of the emulsion.
Disadvantages of biphasic liquid dosage forms:
- Not all drugs are compatible to formulate with biphasic form.
- It is bulky and therefore difficult to handle, transport and store.
- It is costly compared with the solid dosage form.
Difference between monophasic and biphasic liquid dosage form:
Liquid oral doses comprise a wide range of dosage forms, broadly classified as the monophasic and biphasic liquid dosage forms. The major difference between monophasic and biphasic liquid dosage forms is that the monophasic liquid dosage forms have a single homogeneous phase, whereas biphasic liquid dosage forms consist of two distinct phases. Both forms include at least one drug in formulation, monophasic forms are homogeneous and completely dissolve in liquid, whereas biphasic forms are not dissolved in a vehicle.
Commonly asked questions on dosage forms are as follows.
What is the classification of dosage forms?
Pharmaceutical dosage forms are classified based on physical form and route of administration, based on the physical form are solid, semi-solid, liquid, and gaseous dosage form.
How many types of emulsions are there?
There are two basic types of emulsions, oil in a water type (o/w) and, water in an oil type (w/o).
How many types of suspension are there in the pharmacy?
Based on the ratio of solids, suspension dosage forms are empirically classified as dilute or concentrated systems and based on the nature and behavior of solids, suspensions are classified as flocculated or deflocculated.