Learn about the assay of sodium chloride through a laboratory experiment or practical.
Aim:
To determine the percentage purity of given sample of sodium chloride using standard 0.1 N AgNO3 (Volhard’s method).
Requirements:
Glasswares: Burette, burette stand, conical flask, volumetric pipette, beaker, volumetric flask, funnel, glass rod, and wash bottle, etc.
Chemicals: LR grade silver nitrate (AgNO3), sodium chloride (NaCl), and potassium chromate (K2CrO4), etc.
Apparatus: Digital/analytical balance, and Ultrasonicator.
This practical is divided into two parts.
A. Preparation and standardization of silver nitrate (0.1 M)
B. To perform the assay of sodium chloride
Principle of assay of sodium chloride:
We already mentioned the principle of assay of sodium chloride in previous article (
click here to visit).
Preparation and standardization of silver nitrate (0.1 M):
Click here to get the procedure of preparation and standardization of 0.1 M silver nitrate solution.
Preparation of sodium chloride solution (Sample):
Take 01 gm of sodium chloride and dissolve in 50 ml of distilled water in a volumetric flask, and properly mixing it. Once it has completely dissolved, make up the volume to 100 ml.
Titration procedure:
- All glassware should be cleaned and dried according to standard laboratory procedures.
- Before filling the burette for the titration, rinse it with distilled water and then pre-rinse it with a portion of the titrant solution. Pre-rinsing is required to make sure that all solution in the burette is the desired solution, not a contaminated or diluted solution.
- Take the unknown stock solution of titrant in a clean and dry beaker then fill the burette using the funnel.
- Remove air bubbles from the burette and adjust the reading to zero.
- Take 10.00 ml of prepared sample solution of sodium chloride and pour it into a conical flask.
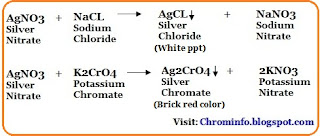
- Add 2-3 drops of potassium chromate solution as an indicator.
- Titrate the sample solution with silver nitrate solution until the endpoint is reached.
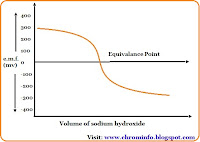
- The actual endpoint of the titration is indicated by a brick red color at the end of the reaction.
- To get accurate results, repeat the titration three times.
- Properly record the readings of the burette.
- Take their mean and calculate the molarity of the silver nitrate solution.
Observation table:
|
Sr. No.
|
Content
in conical flask
|
Burette
reading
|
Volume
of titrant used (ml)
|
|
Initial
|
Final
|
|
1
|
|
|
|
|
|
2
|
|
|
|
|
3
|
|
|
|
|
Mean:
|
Calculations:
V x E x AM x 100 / W x RM
Where,
V is a volume of silver nitrate used
E is an equivalent factor
AM is an actual molarity
RM is a required molarity
W is the weight of the sample
For 1 ml of 0.1 M silver nitrate, the equivalent factor of PHP is 0.005845
Result:
The percentage purity of the sodium chloride (NaCl) sample was found to be_____.
Commonly asked questions on titration are as follows.
Which method is used for assay analysis of sodium chloride?
Assay analysis of sodium chloride is performed using the argentometric titration which is a type of titration involving the silver (I) ion, generally used to determine the amount of chloride present in the sample solution.
Which indicator is used in the assay of sodium chloride?
Assay of silver nitrate is based on the precipitation titration in which potassium chromate solution is used as an indicator that produces a brick red color at the end of the reaction.
Which titrant is used for the assay of sodium chloride?
The titrant in the sodium chloride assay is silver nitrate, which determines the chloride ion concentration. The precipitate of silver chloride is produced in the solution when silver nitrate is slowly.