Titration is a method of quantitative chemical analysis that has several applications in the pharmaceutical field, real-life, daily life, food industry, research, cosmetic industry, wine industry, automotive industry, biochemical and clinical, etc.
Titration, also known as titrimetry, is a typical quantitative chemical analysis used to determine the unknown concentration of an identified analyte.
It is also referring as volumetric analysis because volume measurement is important in titration. In this method, the titrant is added from a burette until the reaction is finished, and an indicator is normally used to indicate the endpoint or equivalence point when the reaction is finished.
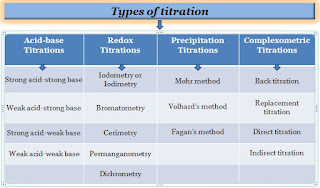
There are several types of titrations including acid-base, complexometric, precipitation, and redox titration with different procedures and goals. However, the most common types of qualitative titration are acid-base and redox titrations.
As advantages concern, it has a simple practical procedure and does not require expensive chemicals and apparatus. Burette, pipette, conical flask, beaker, funnel, volumetric flask, burette stand, and wash bottle, etc. are required apparatus for titrations.
Applications of titration:
- The major application of titration is that it is used to determine the unknown concentration of solute in different fields.
- Pharmaceutical application is the most common application of titration, in which it is used for purity analysis, and content analysis of dosage forms or medicines.
- Wine manufacturers use titration to improve flavor and maintain consistency of product, in the absence of analytical techniques such as gas chromatography (GC) or liquid chromatography (HPLC).
- Titration is used for determining the amount of contamination in water, and determine the correct pH of the water.
- In the cosmetics industry, titration is used to determine the concentration and amount of chemicals to utilize in their products.
- It is used in the food industries to determine the nutritional value of food to maintain the quality of a product.
- The titration process is used to determining the pH and acidity of the initial milk used to manufacture the cheese.
- Titration can also be used to determine the amount of unsaturated and saturated fatty acids in food.
- Titration is used in the manufacturing of biodiesel fuel in the automotive sector.
- The titration process is used in the paint industry to determine chemical concentrations, pH levels, and the amount of water used in paints.
- In the diagnosis of diabetes, titration can be used to determine glucose levels.
- Titration is a procedure commonly used to determine which compounds are present in urine samples.
- The theory and practice of titration are taught in many schools and colleges as part of chemistry courses.
- The Titration process is of importance for environmental studies and treatment of acidic mine waters.
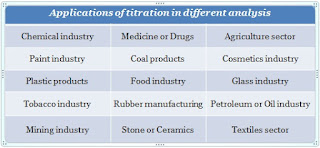
Applications of titration in different fields are listed below:
|
Agriculture
sector
|
Tobacco
industry
|
Chemical
industry
|
|
Paint
industry
|
Coal
products
|
Cosmetics
industry
|
|
Plastic
products
|
Food
industry
|
Glass
industry
|
|
Medicine
or drugs analysis
|
Rubber
manufacturing
|
Petroleum
or Oil Industry
|
|
Mining
industry
|
Stone
or ceramics
|
Textiles
sector
|
What are titrations and what are they used for?
The following are the various types of titrations and their applications.
Application of acid-base titration:
An acid-base titration is a technique for determining an acid's or base's concentration (unknown) by neutralizing it with a known concentration of acid or base.
Application of complexometric titration:
Complexometric titrations are particularly used for determining the concentration of different metal ions in a mixture of sample solutions.
Application of precipitation titration:
It is a type of titration used to determine chloride by using silver ions, in which the analyte and titrant react to form a precipitate during the process of titration.
Application of redox titration:
The redox titration is also called oxidation-reduction titration, it is based on the oxidation-reduction reaction between the analyte and titrant. It is used for the analysis of organic analytes and mainly evaluating chlorination.
Application of non-aqueous titration:
Non-aqueous titration is a type of titration in which the analyte component is dissolved in a non-water-containing solvent. Non-aqueous titration is carried out for water-insoluble drugs, weakly acidic drugs, and weakly basic drugs, etc.
Application of back titration:
It is a titration method that involves reacting an analyte with a known amount of extra reagent to determine its concentration. Back titration is intended to solve some of the issues that may arise when using forward or direct titration.
Application of neutralization titration:
Neutralization is the reaction between an acid and a base used to determine the concentrations of solutes. Acids, bases, and any species that may be converted into an acid or base are among the most commonly studied species.
Application of iodometric titration:
It is known as Iodometry titration, which is a method of volumetric chemical analysis. Iodometric titration method used for determining the concentration of an oxidizing agent in a solution of water samples.
Application of argentometry titration:
In analytical chemistry, argentometry is a type of titration which is based on the precipitation of silver (I) ion compounds. Argentometry titrations are commonly used to find the concentration of chloride in a sample.
Application of Karl Fischer titration:
The Karl Fischer titration is based on the Bunsen reaction between iodine and sulfur dioxide in an aqueous medium that uses volumetric or coulometric titrations to determine the amount of water present in a given analyte.
It is a widely used method in the different pharmaceutical as well as other industries for the determination of moisture content by quality-control analysis for liquid, solid, and gaseous samples. In this method, a Karl fisher apparatus is used.
Application of potentiometric titration:
Potentiometry is a method of determining the concentration of a solute in a given solution by measuring the potential between two electrodes. It is similar to the direct titration of a redox reaction, in this method Instead of using a chemical indicator, the electric potential across the material is detected.
It is particularly used in the characterization of acids, as well as for analysis of metals, elements, and food processing, etc. Generally, it is performed using the potentiometer or pH meter.
Application of spectrophotometric titration:
Spectrophotometry is a method of determining the quantity of a sample by adding known increments of titrant until the end-point is achieved. It is a method of measuring how much light a chemical substance absorbs or transmits, by measuring the intensity of light as a beam of light passes through a sample solution.
Spectrophotometry is one of the most widely used quantitative analysis methods for the determination of analyte concentration using the Beer-Lambert law in a variety of fields such as chemistry, biochemistry, chemical engineering, biology, physics, clinical applications, industrial applications, and research, etc. Generally, it is performed using the UV-visible spectrophotometer or IR-spectrophotometer.
Application of conductometric titration:
Conductometric titration is an analytical technique in which the reaction mixture's electrolytic conductivity is continually calculated. Similar to potentiometry, and spectrophotometry, conductometry titrations also do not require an indicator.
It is performed to determine the electrical conductivity of the sample solution, in the reaction process there is no need indicator. In this titration, a conductivity meter is used to determine the conductance.
Commonly asked questions on titration are as follows.
What is the importance of titration?
Titration is important in pharmaceutical analysis, especially in chemistry, as it allows for the precise determination of solute concentrations.
What are the examples of indicators used in different titrations?
Phenolphthalein, methyl red, methyl orange, methyl violet, methyl yellow, bromothymol blue, litmus, eriochrome black T, sodium diphenylamine, starch indicator, thymol blue, crystal violet, and p-nitrophenol, are some of the indicators used in different titration methods according to the
Which instruments are used for titration?
Nowadays the different types of instruments, such as Automatic titrator, pH meter, Conductivity meter, Karl Fischer titrator, Potentiometric titrator, Thermometric titrator, Isothermal titration, Aalorimeter and an Amperometry, etc. are being used in several industries to precisely execute titration.
People also ask
Uses of titration
Applications of titration curves
Some applications of titration class 10, 11, 12
Applications of non-aqueous titration in pharmacy
Industrial applications of titration
Real-world applications of titrations
Practical applications of titrations
How is titration used in everyday life?
Importance of titration