Learn how to make different concentrations of molar and normal acetic acid solutions, which are needed for many applications such as research, practical, pharmaceutical, chemical laboratory, and industries, etc.
Acetic acid is also known as ethanolic acid because it contains two carbon atoms.
The molecular weight of acetic acid (CHC3OOH) is 60.052 g/mol
The boiling point of acetic acid (CH3COOH) is 118 °C
The density of acetic acid (CH3COOH) is 1.05 g/cm³
The specific gravity of concentrated acetic acid (CH3COOH) is 1.055 g/ml
Generally, a liquid form of acetic acid (CH3COOH) in different concentrations is supplied in the market by vendors.
Requirements of glassware and apparatus:
Digital balance, beaker, pipette, pipette bulb, volumetric flask, measuring cylinder, glass rod, distilled water, AR/LR grade acetic acid, etc.
Calculation method:
Molarity = gram moles of the sample (CH3COOH) / volume of the solution
For example,
We calculate for the concentration of 0.1m acetic acid, for this purpose we can use acetic acid, glacial (99.5 %, 17.4 M). Acetic acid CH3COOH is monoprotic, therefore 0.1N = 0.1M.
We know the molar mass of acetic acid is= (12+3+12+32+1) = 60g/mol.
Molarity = gm moles of the solute (CH3COOH) / volume of the solution
0.1 x volume of the solution = gm moles of the solute (CH3COOH)
gm moles of CH3COOH = 0.1 x 1 = 0.1
gm moles of CH3COOH = gm moles of CH3COOH taken / mol. moles of CHC3OOH
gm moles of CH3COOH = 0.1 = gm of CH3COOH taken / 60.05
gm of CH3COOH taken =0.1 x 60.05
= 6.005 gm
The density of acetic acid is 1.049 g/ml, so 0.1 mol of HOAc (6.01 g) is 5.72 ml.
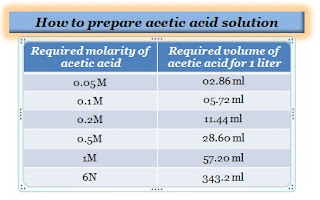
How to prepare 0.05M acetic acid?
Take 02.86 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 0.1M acetic acid solution?
Take 6.005 gm (05.72 ml) of glacial acetic acid using a pipette, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 0.2M acetic acid?
Take 11.44 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 250 ml of 0.25M acetic acid?
Take 03.58 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 250 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 0.5M acetic acid?
Take 28.60 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 1M acetic acid?
Take 57.20 ml of glacial acetic acid, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 2M acetic acid?
Take 114.40 ml of glacial acetic acid, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How to prepare 5N acetic acid?
Take 286.0 ml of glacial acetic acid, slowly and carefully dilute to 01 liter of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How do you make a 6N solution of acetic acid?
Slowly and carefully dissolve 360 g or 343.2 ml of acetic acid in 1 liter of water to make a 6N solution.
How do you make a 2% dilute acetic acid solution?
Take 02.00 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 100 ml of distilled water in a volumetric flask, and properly mix it.
How do you make a 5% dilute acetic acid?
Take 05.00 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 100 ml of distilled water in a volumetric flask, and properly mix it.
How to prepare 10% acetic acid?
Take 10.00 ml of glacial acetic acid using a pipette, slowly and carefully dilute to 100 ml of distilled water in a volumetric flask, and properly mix it.
How to prepare 100 mM acetic acid (sodium) buffer solution (pH=4.7)?
Take 02.87 ml of glacial acetic acid (99.5 %, 17.4 mol/L) and 06.80 gm of sodium acetate trihydrate (M.W.=136.08) and carefully dilute to 500 ml of distilled water in a volumetric flask. Once it has completely dissolved, make up the volume to 1000 ml.
Note:
- Acetic acid is an extremely dangerous acid upon contact. Therefore follow laboratory safety measures (SOP) and please use extreme caution when preparing the solution concentrations.
- When making acid solutions, it is recommended that always add (gradually) acid to water.
- Stir a little amount of acetic acid into a large volume of water at a time, and then dilute the solution.
- When handling acetic acid, always wear a chemical-resistant apron, gloves, and goggles to protect your eyes and skin.
- Since fumes of concentrated acetic acid are toxic when inhaled, they should always be handled in a fume hood.
- Acid should be kept in a special cabinet made of wood. Since metal corrode rapidly when exposed to hydrochloric acid vapors, wooden cabinets are better than metal cabinets for acid storage.
- If acid splashes on your skin or in your eyes, wash it off with water for 15 to 20 minutes.
References:
- Wikipedia contributors. (2022, January 6). Acetic acid. In Wikipedia, The Free Encyclopedia. Available Here
- Solution Preparation Guide, Available Here
- Preparing Buffer Solutions, Available Here
- How can i prepare-02 v/v acetic-acid, Available Here
- Acid & Base Normality and Molarity Calculator. Available Here
No comments:
Post a Comment