Learn how to make different concentrations of molar and normal nitric acid solutions, which are needed for many applications such as research, practical, pharmaceutical, chemical laboratory, and industries, etc.
Generally, a liquid form of nitric acid (HNO3) in different concentrations is supplied in the market by vendors.
Nitrate acid is also prepared in the lab by heating the nitrate salt with sulfuric acid (concentrated).
NaNO3 + H2SO4 -> NaHSO4 + HNO3
The molecular weight of HNO3 is 63.01 g/mol
The boiling point of HNO3 is 83 °C
The density of HNO3 is 1.51 g/cm³
The specific gravity of HNO3 is 1.42
Requirements of glassware and apparatus:
Digital balance, beaker, pipette, pipette bulb, volumetric flask, measuring cylinder, glass rod, distilled water, AR/LR grade nitric acid (HNO3), etc.
Calculation method:
Conversion factors: 1000mL = 1L
Nitric Acid (67-70% w/w) has a molarity of approximately 15.6 moles/L
1L contains 15.6 moles, so 1 mole is contained within = 1L / 15.6 moles.
1 mole = 0.0641 liters = 64.1 ml
Mass of 64.1 mL of HNO3:
= 64.1 ml x 1.41 g/ml
= 90.4 gm
Where 1.41g/ml is the approximate density of 67-70% w/w HNO3
To make a 1 molar solution of nitric acid, dilute 90.4 gm or 64.1 ml of nitric acid in 1000 ml of distilled water.
Nitric acid is a monoprotic acid therefore normality is the same as molarity.
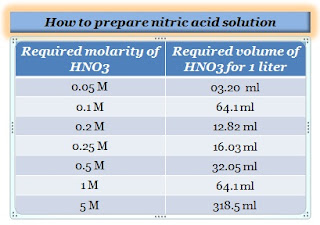
How to prepare 0.05M nitric acid solution?
Take 03.20 ml of nitric acid using a pipette, slowly and carefully dilute to 1000 ml of distilled water in a volumetric flask, and properly mix it.
How to prepare 0.1M nitric acid solution?
To make a 1 M solution, slowly and carefully add 64.1 ml of nitric acid to 250 ml deionized water. Adjust the final volume of solution to 1000 ml with deionized water and properly mix it.
How to prepare 0.2M nitric acid solution?
To make a 0.2 M solution, use a pipette to dilute 12.82 ml of HNO3 to 1000 ml of distilled water in a volumetric flask and thoroughly mix it.
How to prepare 0.25M nitric acid solution?
To make a 0.2 M solution, slowly and carefully add 16.03 ml of nitric acid to 500 ml deionized water. Adjust the final volume of solution to 1000 ml with deionized water and properly mix it.
How to prepare 0.5M nitric acid solution?
To make a 0.5 M solution, slowly and carefully add 32.05 ml of nitric acid to 500 ml deionized water. Adjust the final volume of solution to 1000 ml with deionized water and properly mix it.
How to prepare 1M nitric acid solution?
To make a 1 M solution, slowly and carefully add 64.1 ml of nitric acid to 250 ml deionized water. Adjust the final volume of solution to 1000 ml with deionized water and properly mix it.
2 molar nitric acid solution preparation:
Take 64.1 ml of 67-70% w/w nitric acid slowly and carefully dilute to 500 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
3M nitric acid solution preparation
Take 19.23 ml of 67-70% w/w nitric acid slowly and carefully dilute to 100 ml of distilled water in a volumetric flask. Allow the solution to cool to ambient temperature and properly mix it.
How do you make 4M nitric acid?
Based on a density of 1.413 g/ml, a formula weight of 63.01 g/mol, and a concentration of 70% w/w, our stock solution of HNO3 is calculated to be 15.698 M.
To prepare a 4 M solution, slowly and carefully add 254.82 ml of nitric acid to 700 ml of deionized water. Allow the solution to cool to ambient temperature, make up the volume of solution to 1000 ml with deionized water and properly mix it.
How do you make a 5M HNO3 solution?
Based on a density of 1.413 g/ml, a formula weight of 63.01 g/mol, and a concentration of 70% w/w, our stock solution of HNO3 is calculated to be 15.698 M.
To prepare a 5 M solution, slowly and carefully add 31.85 ml of nitric acid to 70 ml of deionized water. Allow the solution to cool to ambient temperature, make up the volume of solution to 100 ml with deionized water and properly mix it.
How do you make a 6M HNO3 solution?
Based on a density of 1.413 g/ml, a formula weight of 63.01 g/mol, and a concentration of 70% w/w, our stock solution of HNO3 is calculated to be 15.698 M.
To prepare a 6 M solution, slowly and carefully add 38.22 ml of nitric acid to 70 ml of deionized water. Allow the solution to cool to ambient temperature, make up the volume of solution to 100 ml with deionized water and properly mix it.
How to prepare 5% nitric acid solution?
The volume density of 7.14 g of concentrated HNO3 (70%) can be obtained from V = 7.14 g / 1.42 = 5.03 ml.
We can obtain the required 5 percent solution by measuring 5.03 ml of 70% HNO3 and diluting it to 100 ml with water.
How to prepare 10% nitric acid solution?
Take 10.06 ml of 70 percent HNO3 using a pipette, slowly and carefully dilute to 100 ml of distilled water in a volumetric flask, and properly mix it.
Note:
- Nitric acid is an extremely dangerous acid upon contact. Therefore follow laboratory safety measures (SOP) and please use extreme caution when preparing the solution concentrations.
- When making acid solutions, it is recommended that always add (gradually) acid to water.
- Stir a little amount of HNO3 into a large volume of water at a time, and then dilute the solution.
- When handling HNO3, always wear a chemical-resistant apron, gloves, and goggles to protect your eyes and skin.
- Since concentrated nitric acid is toxic when inhaled, it should always be handled in a fume hood.
- Acid should be kept in a special cabinet made of wood. Since metal corrode rapidly when exposed to hydrochloric acid vapors, wooden cabinets are better than metal cabinets for acid storage.
- It has a corrosive effect on the skin, causing painful sores.
- If acid splashes on your skin or in your eyes, wash it off with water for 15 to 20 minutes.
References:
- USP Pharmacopoeia, Nov. 13, 2014
- Wikipedia contributors. (2022, January 14). Nitric acid. In Wikipedia, The Free Encyclopedia, Available Here
- ‘Making Solutions’. SEASTAR CHEMICALS, Available Here
- Acid & Base Normality and Molarity Calculator. Available Here
- SolCalc Help: Preparing 10 % HNO3. Available Here
- Blog Post Westlab. difference between molarity and normality, Available Here
No comments:
Post a Comment