Friability is an evaluation test for tablets used to determine the physical strength of compressed or uncoated tablets when exposed to mechanical shock and attrition.
Dosage forms are the means by which drug molecules are delivered to sites of action within the body. They can be solid, semisolid, liquid, and gaseous forms and can be taken orally, parenterally, sublingually, topically, rectally, and topically, etc. There are many types of dosage forms used to treat such as tablets, capsules, powders, caplets, granules, transdermal patch, plasters, paste, creams, gels, suppository, pessaries, solutions, drops, syrups, linctus, and lotions, etc. however tablets are one of the most commonly used since they offer several advantages.
Tablets are solid unit dosage forms of pharmaceuticals that contain both active pharmaceutical ingredients (API) and excipients and are designed for oral administration. It is normally in powder form and is pressed using a compression machine into a solid dosage. Depending on the amount of therapeutic substance and the desired manner of administration, they are formulated in different sizes, shapes, appearances, tastes, odors, weights, thickness, hardness, disintegration, and dissolution. Depending on the rate of release in the stomach or intestines, several types of tablets can be formulated such as compressed, multiple-compressed, sugar-coated, film-coated, enteric-coated, chewable tablets, etc.
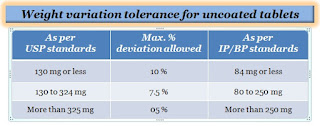
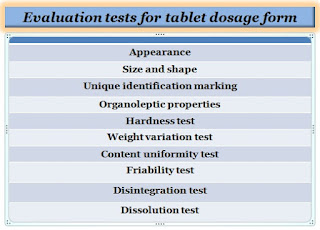
To evaluate tablets there are different quality control test is performed to ensure the quality of the product which is mentioned in pharmacopeias (USP, BP, and IP) such as hardness test, weight variation test, friability test, content uniformity test, disintegration test, and dissolution test, etc. some are the official and some are the non-official.
What is the friability test in tablets?
Friability test is a quality control test for tablet dosage forms as per official standards which are described in the pharmacopeia such as USP which also mentions their standard ranges. Friability testing is a technique for determining the physical strength of uncoated or compressed tablets when they are subjected to mechanical shock and attrition. In other words, friability testing determines how much mechanical stress the tablets can bear during the entire manufacture, delivery, and client handling.
Friability testing has become a widely recognized technique in the pharmaceutical industries and research, and the equipment used to perform this procedure is known as a friability tester or friabilator. It involves repeatedly dropping a tablet sample over a set time, using a drum that rotates with a spin. 10 whole tablets are weighed and placed in the drum of the friabilator where it is rotated. The broken tablets are checked, as well as the percentage of tablet mass lost due to chipping.
Why friability test is performed?
The friability test is performed to determine how much % weight is lost due to mechanical shock during entire manufacturing, transportation, and delivery. Or, it is performed to check the durability of tablets and mass loss of tablet powder through chipping.
How many tablets are used for the friability test?
10 tablets are used to perform the friability test. Take tablets weighing equal to or less than 6.5 grams if the unit weight is equal to or less than 650 milligrams. If a unit weight of tablets exceeds 650 mg, collect 10 tablets as a whole sample.
What types of tablets are used for friability tests?
Friability testing is usually performed using uncoated and compressed tablets.
How do you calculate friability?
The friability value is expressed in %, which is calculated using the below formula.
Friability = W1–W2/W1 X 100, where W1=Weight of the tablet before the test, and W2=Weight of the tablet after the test.
Typically, the test is run only once, however in case of difficulty in the interpretation of the result, or if the final weight loss exceeds the target value, the test may be repeated twice, and the result may be returned as the mean of the three trials. Except for cracked, broken, chipped tablets, the test is not acceptable if the rolled tablet weighs more than 1%.
What is the limit for friability of tablets?
According to the USP, the acceptance criteria after friability testing for 4 minutes or 100 rounds in the apparatus should not exceed 1% of the tablet's weight.
Factors affecting friability of tablets:
The several factors that can affect the friability test such as tablet design, hardness, punches that are in poor condition, binder being used, and moisture content of tablet granules.
Friability test procedure:
- Requirement: Tablet dosage form and friability test apparatus.
- Weigh the number of tablets accurately and complete the procedure as in the individual monograph, if any.
- Ensure the cleaning and power connection of the instrument.
- Before starting operation, check the calibration status of the apparatus.
- Note the weight and transfer the required amount of tablets into the drum.
- Set all test parameters such as revolution count (100 rounds/ 4 min) and time and start testing.
- Remove the sample from the drum, and out dust or powder, and re-weigh them.
- Subtract the weight from the starting weight to calculate the loss.
- Now calculate the friability by applying the formula.
- If the result is outside the acceptable limits, repeat the test three times and determine the mean.
- Clean the device thoroughly and turn it off.
You may also like this