Creams and ointments are both semisolid dosage forms intended for topical application, it may be medicinal or cosmetic. The major difference between cream and ointment is the ratio between oil and water used in the formulation, the cream contains 50% oil and 50% water whereas the ointment contains 80% oil and 20% water.
Dosage forms are the means by which active pharmaceutical ingredients (API) are delivered to the specific site of the body. Based on the physical form they are solid, liquid, semi-solid, and gaseous dosage forms, and based on the route of drug administration they are topical, oral, parenteral (Injection), inhalation, and some are installed in the body cavities. Some examples of dosage forms are tablets, capsules, injections, syrup, solutions, lotions, liniments, throat paints, aerosols, and sprays, etc.
| Table of Contents |
A topical administration is applied to a specific place on or within the body. Often the topical route means cosmetic use or treatment of diseases through a variety of classes, including gels, creams, pastes, emulsions, lotions, liniments, foams, and ointments on body surfaces such as the skin, mucous membranes.
Creams:
The cream is a semi-solid emulsion made from a combination of 50% oil and 50% water used for medicinal as well as cosmetic purposes. It is non-greasy, light texture disappears on rubbing, and is quickly absorbed by our skin. Creams are water-soluble, so they wash off quickly. It can also be used to treat rashes, allergies, or any other skin-related issues.
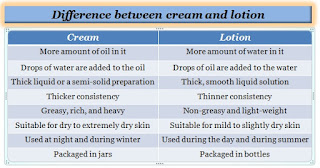
Creams are similar to lotions both are made with a mixture of oil and water which helps skin to soften, moisturize, and cleanse. Creams are suitable for dry skin and lotions are suitable for mild to slightly dry skin.
Ointments:
Ointments are homogenous semisolid dosage forms for external (local) use, intended for application to the skin or certain mucous membrane. It is an oil-based and preservative-free product that contains 80% oil and 20% water. Ointments are a good absorbent and offer an occlusive barrier on the skin which prevents loss of moisture and providing the maximum advantages.
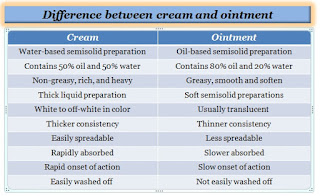
Difference between creams and ointments:
Ointment and cream both are types of topical preparations which are used for the cosmetics and pharmaceutical. However, they have some differences based on nature and properties.
- Creams are made up of more than 20% water and volatiles, whereas ointments are made up of less than 20% water and volatiles.
- Creams are opaque, and relatively soft, while ointments are smooth and soften.
- The creams are generally white to off-white, while the ointments are translucent.
- Pharmaceutical creams can be easily spread on the skin, while ointments may be less spreadable.
- The cream has a short duration of action, while the ointment has a prolonged action compared to cream.
- In comparison to ointments, creams are non-greasy, while ointments are greasy.
- The cream is a water-based formulation whereas ointment is an oil-based semisolid preparation.
- In comparison to ointment, the cream is thinner, while ointment is thicker.
- Creams are absorbed faster, while ointments take longer to be absorbed through the skin.
- The shelf-life of the cream is short, while the shelf-life of the ointment is longer.
- The cream has a faster onset of action, while the ointment has a slower onset of action than the cream.
- Creams are easily washed off as they are non-greasy, while ointments are not easily washed off.
- Creams do not leave stains on clothing, whereas ointments can leave stains on clothing.
Ointment VS cream
The similarity between ointments and creams:
The ointments and creams both are semisolid preparation for topical use that contains oil and water as a base. The similarity between ointments and creams is that both are used for cosmetics as well as medicinal purposes.
Commonly asked questions on ointments and creams are as follows.
Why are creams preferred over ointments?
Creams are suitable for dry skin and it works better on large areas of skin as it spreads more easily than ointments.
What are ointments used for?
Ointments, which contain moisturizers, medications, and cosmetics, can be applied to the skin, and mucus membranes to help treat bites, cuts, burns, scrapes, and hemorrhoids.
You may also like this