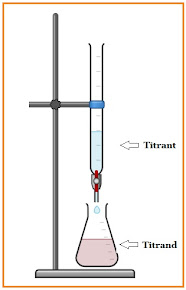
Titration is a typical quantitative chemical analysis method used in laboratories to quantify the concentration of a particular analyte. Titration, also known as titrimetry or volumetric analysis, is a method in which the titrant is added from a burette until the reaction is complete, and an indicator is usually employed to mark the endpoint of the reaction.
There are different types of titration such as acid-base, redox, precipitation, and complexometric titrations, however, in quantitative chemical analysis, redox titration and acid-base titration are most commonly used.
What is acid-base titration with example?
An acid-base titration is a quantitative analysis method used to determine the concentration of an acid or base by precisely neutralizing acid or base with a known concentration standard solution, in which a pH indicator to monitor the reaction.
The concentration of a solution (molarity) of an analyte solution can be determined if the acid dissociation constant (pKa) of the acid or the base dissociation constant (pKb) of the base is known. If the solute solution has a known solution concentration, the pKa can be calculated by generating a titration curve.
Principle of acid-base titration:
As described in the theory of acid-base titration, the principle involves using a burette and pipette along with a pH indicator to determine the concentration of an acid or basic.Usually, as in titration, a neutralization reaction occurs between acid and base, hydroxide ions and hydrogen proton, and water is formed. An indicator is a dye added to have to be change color, whose color depends on the pH of the solution.
It is dissolved in the sample solution and is often used to detect the endpoint of the titration which is also known as the equivalence point, where the color change occurs.
Indicators used in acid-base titration:
The pH range of an indicator is the most essential property, which is reliant on the acid strength of the indicator. The pH range of an indicator is the range of pH values across which the indicator changes color from acid to base.
It ranges from the highest pH, where only the acid form can be seen, to the lowest pH, where only the base form can be seen. Since the indicator does not change color at certain pH levels, it is not sensitive to pH changes outside of its range.
Acid-base indicators are generally classified into below listed three groups.
1. The phthaleins and sulphophthaleins: example-phenolphthalein indicator
2. Azo indicators: example- methyl orange indicator
3. Triphenylmethane indicators: example- malachite green indicator
Selection of indicator in acid-base titration:
In acid-base titrations, different indicators are used. The selection of indicators depends on the type of titration and whose pH range falls within the pH change of the reaction.
- Strong acid-strong base: The phenolphthalein is generally preferred due to color change seen more easily.
- Weak acid-strong base: Phenolphthalein is more proffered for this titration because it changes sharply at the equivalence point.
- Strong acid-weak base: Methyl orange is more proffered for this titration because it changes sharply at the equivalence point.
- Weak acid-weak base: Because a vertical portion of the curve above two pH units is required, there is no indication is suitable for this titration.
Types of acid-base titration with their examples:
The acid-base titration is classified into four different types such as strong acid-strong base, weak acid-strong base, strong acid-weak base, and weak acid-weak base.
Strong acid-strong base:
As an experimental concern, it is one of the easiest titrations to perform among the four forms of acid-base titrations. It involves the complete dissociation of a strong acid and a strong base in water, resulting in a strong acid-strong base neutralization reaction. When the moles of acid and base are the same and the pH is 07.00, it reaches the equivalence point.
Weak acid-strong base:
In this type of titration, the protons are direct transfer from the weak acid to the hydroxide ion. In the reaction of a weak acid (acetic acid) with a strong base (NaOH), the acid and base react in a one-to-one ratio. At the equivalence point of a weak acid–strong base titration, the pH is larger than 07.00.
Strong acid-weak base:
In this type of titration, the acid and base will react to form an acidic solution. Throughout the titration, a conjugate acid is formed, which subsequently reacts with water to form hydronium ions. At the equivalence point of a strong acid-weak base titration, the pH is less than 07.00.
Weak acid-weak base:
Unlike strong acids and strong bases, the shape of a weak acid's or base's titration curve significantly depends on the acid's or base's identity and the associated acid ionization constant (Ka) or base ionization constant (Kb) (Kb). In the titration of a weak acid or a weak base, the pH also changes much more gradually around the equivalence point, which is greater or less than 07.00, respectively.
Examples:
- Hydrochloric acid (HCl) and sulphuric acid (H2SO4) are two examples of strong acids.
- Acetic acid (CH3COOH) and formic acid (CH2O2) are the two examples of weak acids.
- Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are two examples of strong bases.
- Ammonia and methylamine are the two examples of weak bases.
Applications of acid-base titration:
- Acid-base titrations are most commonly used to determine the unknown acid or base concentration of the analyte.
- It is used as a quantitative chemical analysis.
- It has the potential to be used in pharmaceutical applications.
- It can be used in environmental analysis.
- It is used to determine the barbiturates, aspirin, and amino acid.
Experimental procedure of acid-base titration:
- Requirements: Conical flask, funnel, beaker, pipette, burette, burette stand, spatula, wash bottle, indicator, unknown solution, and standard solution.
- Titration procedure:
- Clean and dry all the glassware’s with distilled water and rinse the burette with the standard solution.
- Fill the burette with a standardized solution, accurately measure the volume of the analyte, and add in the conical flask, also add a few drops of indicator using the pipette.
- Titrate it with the standardized solution until the indicator changes the color. When the indicator permanently changes the color, the endpoint reaches.
- Repeat the titration at least three more times and record the initial and final readings in the observation table and calculate the value.
Commonly asked quetions on acid-base titration are as follows.
What are the three theories of acids and bases?
Arrhenius concept of acid and base, Bronsted-Lowry concept and Lewis concept are the three theories that identify a singular characteristic which defines an acid and a base.
Why phenolphthalein is used in acid-base titration?
In a strong acid-strong base titration a phenolphthalein indicator is chosen since it changes color in a pH range of 8.3 to 10.
Why is acid-base titration important?
The objective of a acid-base titration is to find out the concentration of an acid solution by titrating it with a known concentration of a basic solution, or vice versa until neutralization occurs.
Which indicator is used in acid-base titration?
Phenolphthalein, thymol blue, methyl orange, methyl yellow, methyl red, and phenol red, etc. are some of the examples of indicators used in acid-base titration.
People also ask
What happens in acid-base titration?
How does acid-base titration work?
How to choose an indicator for an acid-base titration
How do you perform acid-base titration?
What is the titration formula?
What is acid-base titration for kids?
Acid-base titration class 11
Advantages of acid-base titration