Learn about the estimation of hardness of water by edta complexometric method through a laboratory experiment or practical.
Complexometric titration is also known as chelatometry titration, which is a type of volumetric analysis useful for determining different metal ions in which the formation of a colored complex detects the endpoint. Direct, back, and replacement titration are the types of complexometric titration.
Aim:
To determine the total hardness of the water sample by the EDTA complexometric titration.
Requirements for water hardness test:
Glasswares: Burette, burette stand, conical flask, volumetric pipette, beaker, volumetric flask, funnel, glass rod, wash bottle, etc.
Chemicals: LR grade ethylenediaminetetraacetic acid (C10H16N2O8), ammonia (NH3), hydroxylamine hydrochloride (HONH2•HCl), ethyl alcohol (C2H5OH), ammonium chloride (NH4Cl), magnesium bicarbonate (Mg (HCO3)2), and erichrome black-T indicator (C20H12N3O7SNa), etc.
Apparatus: Digital/analytical balance.
Suitable for test samples:
Drinking water, wastewater, cooling water, environmental water, raw water, process water, surface water, boiler water, etc.
Principle for estimation of hardness of water:
The hardness of water is due to the presence of dissolved calcium and magnesium salts. It is unsuitable for drinking, bathing, and washing, and causes scale formation in boilers. As a result, it is necessary to estimate the amount of hardness-causing chemicals in a water sample. Once determined, the amount of chemicals needed for water treatment can be calculated.
Using complexometric titration, the hardness is determined. The hardness of water is estimated by titrating with a standard solution of the complexing agent ethylene diamine tetra acetic acid (EDTA). As EDTA is insoluble in water, its disodium form is used in this experiment. EDTA can form four or six coordination bonds with a metal ion.
Total hardness:
The total hardness is caused by the presence of chlorides, bicarbonates, and sulphates of calcium and magnesium ions.
Temporary hardness:
Temporary hardness is caused by the presence of bicarbonates of magnesium and calcium ions. It can be easily eliminated through boiling.
Permanent hardness:
Permanent hardness is caused by the existence of sulphates and chlorides of magnesium and calcium ions. It cannot be eliminated through boiling.
Preparation of reagents:
Preparation of EDTA solution:
Take 04.00 gm of ethylenediaminetetraacetic acid and 0.1 gm of magnesium bicarbonate and dissolve them in 500 ml of distilled water in a volumetric flask, and properly mix it. Once it has completely dissolved, make up the volume to 1000 ml.
Preparation of eriochrome black-T indicator solution:
Take 0.4 gm of eriochrome black-T and 4.5 gm of hydroxylamine hydrochloride and dissolve them in 50 ml of 95% ethyl alcohol in a volumetric flask, and properly mix it. Once it has completely dissolved, make up the volume to 100 ml.
Preparation of ammonia buffer solution:
- A: Take 16.9 gm of ammonium chloride and pour in 143.0 ml of ammonia solution.
- B: Take 01.25 gm of magnesium salt of EDTA and dissolve it in 50 ml of distilled water.
Mix the A and B stock solutions and dilute to a volume of 250 ml with distilled water. Dilute 10.00 ml of the prepared solution with distilled water.
Procedure for determination of hardness of water by EDTA titration:
- All glassware should be cleaned and dried according to standard laboratory procedures.
- Before filling the burette for the titration, rinse it with distilled water and then pre-rinse it with a portion of the titrant solution. Pre-rinsing is required to make sure that all solution in the burette is the desired solution, not a contaminated or diluted solution.
- Take the standard EDTA solution (titrant) in a clean and dry beaker then fill the burette using the funnel.
- Remove air bubbles from the burette and adjust the reading to zero.
- Using a pipette, 50 ml of sample water and pour into a conical flask.
- If the sample has a large amount of metal ions, use a lesser volume and dilute it to 50 ml.
- Add 1ml prepared solution of ammonia buffer.
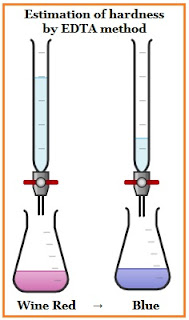
- Then, add 2-4 drops of Eriochrome Black-T Indicator to this solution, which gives the solution a wine-red color.
- Start the titration by adding EDTA drop by drop followed by stirring continuously, until the solution turns from wine red to blue color.
- Repeat the titration three times to get accurate results, properly record the readings of the burette and calculate.
- Take a 50.00 ml sample and boil it in another flask. (Add distilled water to get desired final volume).
Observation table:
|
Sr.
No. |
Content
in conical flask |
Burette
reading |
Volume
of titrant used (ml) |
|
|
Initial |
Final |
|||
|
1 |
|
|
|
|
|
2 |
|
|
|
|
|
3 |
|
|
|
|
|
|
Mean: |
|||
Result:
The collected water sample contains total hardness = _____ppm
You may also like this:
No comments:
Post a Comment