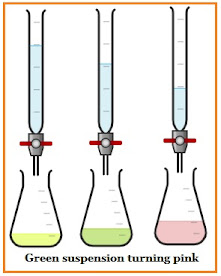
Back titration or indirect titration is a type of complexometric titration in which a known excess of standard reagent is introduced to the solution and the excess is titrated instead of the original sample.
Back titration is a titration performs in reverse. In a back titration, two steps are commonly followed: measuring the amount consumed by the analyte and calculating the excess quantity.
Why is back titration used?
When the endpoint of the reverse titration is easier to identify than the endpoint of the regular titration, such as in precipitation reactions, back titration is useful. Back titrations are also effective when the interaction between the analyte and the titrant is very slow, or when the analyte is in a non-soluble solid.
| Table of Contents: |
When do we use back titration?
- Back titration is performed when the molar concentration of an excess reactant is known, but we need to determine the concentration of an analyte.
- When the analyte and titrant reaction is slow or when the analyte is in an insoluble solid
- When determining the endpoint of a normal titration is difficult, such as weak acid and weak base titration.
- It is used when the acid or base is a salt that is insoluble, such as calcium carbonate.
- Back titration is most typically used when determining the endpoint of a regular titration is difficult.
- When one of the reactants is volatile, such as ammonia.
Why is back titration used in EDTA?
Titrations with EDTA can be done in different ways, including direct, back (indirect), displacement, and masking. Using normal titration procedures isn't always possible. Back titration is a technique that can be used in such instances.
Generally, we used two reagents in back titration: one that reacts with the original sample and the other that reacts with the first reagent. In this method, the excess of EDTA is added to the metal ion solution, which is then titrated with a known concentration of a second metal ion.
With EDTA, the second metal ion must form a weaker complex than the solute ion. As a result, the second metal does not displace the solute ion from its complex with EDTA.
Why do we use back titration in the analysis of aspirin?
For direct titrations, several reactions are slow or have an unfavorable equilibrium. Aspirin is a weak acid with a slow hydrolysis rate; i.e., each molecule of aspirin reacts with 2 hydroxide ions. To overcome this problem, we use back titration in the analysis of aspirin.
In which, an HCl (Hydrochloric acid) titration is used to determine the amount of unreacted base when a known excess amount of base is introduced to the sample solution. This type of reaction occurs at a rapid rate, resulting in an abrupt and easily seen end-point.
Why is back titration used to determine calcium carbonate?
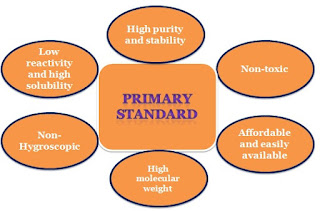
Because calcium carbonate (CaCO3) is insoluble in water and cannot be directly titrated, determining its concentration requires a back titration. Back titration is done when the primary standard is not soluble in water and the direct titration is not possible.
Where is back titration used?
When the molar concentration of an excess reactant is known, but the strength or concentration of an analyte must be determined, back titration is performed. In acid-base titrations, back titration is commonly used: When the acid or base is an insoluble salt (for example calcium carbonate).
Why is back titration more accurate?
In a direct titration, a standard titrant is added to the analyte until the endpoint is reached. In a back titration, an excess of standard titrant is added to the analyte, and the excess titrant is titrated to determine how much is in excess, this makes it a more accurate method.
What is the example of back titration in analytical chemistry?
Determination of acetylsalicylic acid in aspirin and determination of calcium carbonate (CaCO3) is an example of back titration that performs in the lab.
You may also like this: