High purity, ready and cheap availability, stability, non-toxicity, low hygroscopicity, and high equivalent weight are the properties of a good primary standard.
What is a standard?
Standards are materials that contain an exactly known concentration of a substance for use in quantitative analysis. A standard provides a reference that can be used to determine unknown amounts or calibrate analytical instruments. There are two types of analytical standards: primary standards and secondary standards.
What is the primary standard in chemistry?
In chemistry, a primary standard is an exceedingly pure substance (99.9% accurate), which is easy to weigh and gives an indication of the number of moles in a compound. They do not react with the components of the air and maintain their structure for a long period.
They have special chemical and physical properties and are very pure and stable. Primary standards are frequently used in titration studies and other analytical chemistry techniques to determine an unknown concentration of solute.
"A primary standard is a measurement used to calibrate working standards. A primary standard is chosen as of its precision and stability when exposed to other compounds. Metrics such as length, time, and mass can be used to measure primary standards"
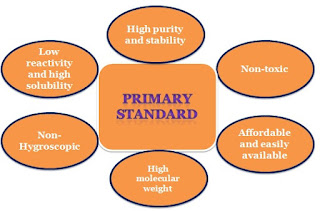
Characteristics of the primary standard:
Properties of primary standard:
It has a high degree of purity
Has a non-toxic or low toxicity
It has low reactivity and high stability
Is affordable and easily available
It has a high equivalent weight
It has a high solubility
Not hygroscopic
Properties of primary standard:
Let’s get a detailed explanation of the characteristics of a good primary standard. A primary standard must meet the following requirements:
- High purity:
It should be available in the purest form and also be preserved in a pure state.
- High stability:
A primary standard must be highly stable. Less stable compounds do not act consistently, making them reactive and unsuitable for use as standards. Even during storage, the composition of a primary standard should remain consistent.
- Non-toxic:
The standard should be a non-toxic chemical. Any material that is a nuisance or aesthetically ineffective and does not pose a health hazard is considered non-toxic.
- Cheap in cost, and readily available:
The standard should be easily accessible and economical as it may usually be employed in a large amount.
- Low reactivity:
The reactivity of the primary standard should be minimal. The results of chemical analysis are affected by reactive substances, which are not employed as primary standards.
- Non-Hygroscopic:
The substance should be stable in the open atmosphere and unmodified in the air, i.e. during weighing.
- High molecular weight:
The relative molecular weight of a primary standard should be high to reduce weighing errors.
- Solubility:
It must be highly soluble in the solvent/ water to make the standard for further process.
Frequently Asked Question (FAQ):
What are some examples of primary standards?
Potassium dichromate (K2Cr2O7), sodium chloride (NaCl), sodium carbonate (Na2CO3), and potassium hydrogen phthalate (KHP) are some of the examples of primary standards which are used in different types of titration.
What is a secondary standard?
A secondary standard is made in the lab for a specific analysis. In chemistry, it is usually standardized against the primary standard. Sodium hydroxide (NaOH) is an example of a secondary standard.
References:
- Wikipedia contributors. "Primary standard." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 26 Oct. 2021.
- Helmenstine, Ph.D. Anne Marie. “Learn About Primary and Secondary Standards in Chemistry.”
- Skoog, Douglas A., Donald M. West and F. James Holler. "Fundamentals of Analytical Chemistry 8th ed." Harcourt Brace College Publishers.
You may also like this:
No comments:
Post a Comment