Learn about the definition, principle, reaction, indicator, endpoint, and advantages of Fajan’s method.
Titration is a method of determining the concentration of an unknown solute using a known concentration solution. In which a titrant is usually applied from a burette to a known compound volume until the reaction is complete, the endpoint is detected using an indicator. Acid-base titration, redox titration, precipitation titration, and complexometric titration are all types of titrations.
| Table of Contents: |
What is precipitation titration?
Precipitation titration is a type of titration in which the titration reaction formed a precipitate. The titrant reacts with the compound to produce an insoluble substance (precipitate). This uses silver ions to find out chloride levels and continues until all compounds have been consumed.
Precipitation titration is a volumetric process based on the formation of a slightly soluble precipitate. On the other hand, the argentometric technique is a precipitation titration method that uses silver nitrate (AgNO3) as the precipitating agent. There are three forms of precipitation titration: Mohr, Vollhard, and Fajan.
Principle involved in precipitation titrations:
The basic principle of precipitation titrations is that the amount of precipitating reagent or precipitant added is equivalent to the substance being precipitated.
Amount of added precipitating agent = the amount of a compound that is precipitated
What is Fajan’s method in chemistry?
The Fajans method is an analytical technique that uses adsorption to detect halide content. This technique was presented by Kazimierz Fajan, a chemist from the United States, for this reason, it is called Fagan's method. It is commonly used to quantitatively analysis of halide ions or thiocyanate ions.
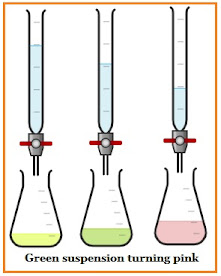
Silver chloride is also known as indication adsorption as chloride ions are adsorbed on its surface. This method uses a dichlorofluorescein indicator. The suspension (of AgCl and indicator) has reached the endpoint when it turns pink from green.
Definition of Fajan’s method:
"Fajan's method is a precipitation titration in which silver ions are titrated with halide/thiocyanate ions in the presence of an adsorption indicator"
Example of Fajans method:
Cl– ions titration using AgN03 in the presence of an adsorption indicator, in which, AgN03 is filled in a burette, and Cl– ion solution with indicator is added in a conical flask.
Reaction involved in Fajans method:
Adsorption indicators work differently than chemical indicators and they can be used in a different precipitation titration, not simply argentometric ones. Assume we want to analyze Cl- in a sample solution by titrating with Ag+. The titration reaction would be
Ag+ + Cl− ⇌ AgCl(s)
Colloidal particles are formed by silver chloride. Because of the adsorption of excess Cl- to the surface of the precipitant particles before the equivalence point, the surface of the precipitant particles will be negatively charged.
Indicator used in Fajan’s method:
The dye dichlorofluorescein can be used as an indicator to determine chloride in Fajan's titration, by which the endpoint is detected by the green suspension turning pink.
Fajan’s method endpoint:
When the anionic indicator becomes adsorbed on the cationic particles of the precipitate, the green suspension turns pink, this is the endpoint in Fajans titration. The indicator for the titration of Cl- with Ag+ is dichlorofluorescein.
Frequently Asked Question (FAQ):
Which titrant is used in the Fajan method?
In the Fajans method for Cl-, Ag+ is used as a titrant. This method is used to detect the concentration of chloride is in a sample. The sample solution is titrated against a known concentration of silver nitrate (AgNO3) solution. When AgNO3 is added to a solution containing chloride ions results in the formation of a finely divided white precipitate AgCl.
What is the method used in Fagan’s method?
The indicator absorption method is used for Fajan's method.
Which titration is known as argentometric titration?
The titrations with silver nitrate are known as argentometric titration. This titration is used to determine the concentrations of chloride, bromide, and cyanide ions. As an indicator, sodium chromate is used.
What is Fajans rule give one example?
According to Fagan's rule, a compound with a low positive charge, a large cation, and a small anion form an ionic bond, whereas a compound with a high positive charge, a small cation, and a large anion form a covalent link. For example, an aluminum atom with a +3 charge, has a greater positive charge.
References:
- Wikipedia contributors. "Argentometry." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, Available Here:
- Wikipedia contributors. (2022, March 9). Fajans' rules. In Wikipedia, The Free Encyclopedia. Available Here:
- ‘Precipitation Titration’. Chemistry LibreTexts, 23 Nov. 2014, Available Here:
You may also like this:
No comments:
Post a Comment