Learn about the characteristics, mechanism, examples, and types of redox indicators that are used to detect the endpoint of redox reactions by different ways such as self indicators, redox indicators, external indicators, and instrumental techniques, etc.
Titration, also known as titration, is a volumetric analysis used to determine the concentration of an analyte in a sample solution in the presence of an indicator. Based on the reaction and the goals there are four types of titration: acid-base, complexometric, redox, and precipitation.
A solute is titrated by placing a burette containing a titrant with a known concentration over a conical flask or beaker containing the solute. Titrant is added until the reaction is complete; typically, an indicator indicates an endpoint or an equivalence point of the reaction.
| Table of Contents: |
What is redox titration?
Redox titration is a method used in the laboratory to determine the concentration of an analyte by carrying out a redox reaction between the analyte and the titrant. Redox titration depends on the oxidation-reduction reaction that occurs between the analyte and the titrant.
In order to evaluating the redox titrations, it is necessary to obtain the shape of the titration curve that corresponds. In this type of titration, it is much easier to monitoring the concentration of the reaction potential rather than the concentration of the reacting species.
Depending on the titrant used, there are different types of redox titration, such as bromatometry, cerimetry, permanganometry, iodometry or iodimetry, dichrometry, etc., and depending on the method it is classified as direct titration, and back titration.
What is redox indicator in chemistry?
An indicator that exhibits a definite color change at a specific electrode potential is referred to as a redox indicator. Due to the need for a rapid and reversible change in color, the oxidation-reduction equilibrium of an indicator redox system needs to be established very rapidly.
Redox indicators are organic molecules can be either oxidized or reduced, and their oxidized and reduced forms exhibit different colors.
In ox + ne ↔ In red
Example of redox indicator:
A good example of a redox indicator is 2, 2’-bipyridine, it is an organic compound with the formula C10H8N2 that changes color from pale blue to red when in solution at an electrode potential of 0.97 V.
Types of redox indicators:
There are two common types of redox indicators that are used in chemistry are metal complexes of phenanthroline and bipyridine, and organic redox systems.
- In the systems of metal complexes of phenanthroline and bipyridine the metal changes oxidation state.
- In the systems of organic redox (methylene blue) systems a proton participate in the redox reaction. Consequently, redox indicators can also be classified into groups of pH-independent or pH-dependent.
Some commonly used redox indicators are methylene blue, diphenylamine, Ferroin (1, 10- phenanthroline iron (II) sulphate, and nitro ferroin, etc.
Characteristics of redox indicators:
The following are the general characteristics of good redox indicators.
- In a redox titration, a redox indicator should show that the oxidation potential changes rapidly near the equivalence point.
- In a redox titration, a redox indicator should characterize the rapid change in oxidation potential near the equivalence point.
- The ideal redox indicator has an oxidation intermediate midway among the solution titrating and the titrant that need to be produce a sharp, easily noticeable color change.
- When we carry out the redox reaction, both the oxidation and reduction processes need to be quick and reversible.
- When operating a specific potential range, each redox indicator will change color.
- The indicator's standard potential must be at least 0.15V different from the redox system's for a sharp color change at the endpoint.
Mechanism of redox indicator:
Diphenylamine was one of the first redox indicators that were commonly used in titrimetric analysis. Since this molecule is not readily soluble in water and tungstate ion and mercury (II) chloride interfere with its activity, the barium or sodium salt of diphenylamine sulfonic acid is utilized more frequently.
This indicator is available in both reduced and oxidized forms, with the latter having a dark violet color. Using diphenylamine as an example, it has been shown that the mechanism underlying the color shift is as follows:
The diphenylamine solution in concentrated sulphuric acid (H2SO4) is colorless. It is used while titrating Fe (II) in a solution of potassium dichromate (K2Cr2O7). It is initially converted to colorless diphenyl benzidine by an oxidizing agent. This is then further reversibly oxidized to produce diphenyl benzidine violet.
If diphenyl benzidine violet is let to in an excess of dichromate solution, it will oxidize further. The subsequent oxidation is irreversible, and red or yellow compounds of unknown composition are formed.
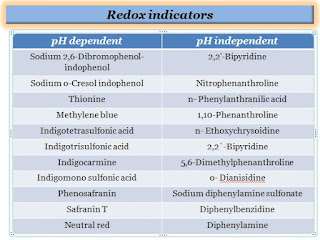
List of redox indicators:
pH dependent
pH independent
Sodium 2,6-Dibromophenol-indophenol (+0.64 V/+0.22 V)
2,2'-Bipyridine (+1.33 V)
Sodium o-Cresol indophenol (+0.62 V/+0.19 V)
Nitrophenanthroline (+1.25 V)
Thionine (+0.56 V/+0.06 V)
n- Phenylanthranilic acid (+1.08 V)
Methylene blue (+0.53 V/+0.01 V)
1,10-Phenanthroline (+1.06 V)
Indigotetrasulfonic acid (+0.37 V/-0.05 V)
n- Ethoxychrysoidine (+1.00 V)
Indigotrisulfonic acid (+0.33 V/-0.08 V)
2,2`-Bipyridine (+0.97 V)
Indigocarmine ( +0.29 V/-0.13 V)
5,6-Dimethylphenanthroline (+0.97 V)
Indigomono sulfonic acid (+0.26 V/-0.16 V)
o- Dianisidine (+0.85 V)
Phenosafranin (+0.28 V/-0.25 V)
Sodium diphenylamine sulfonate (+0.84 V)
Safranin T (+0.24 V/-0.29 V)
Diphenylbenzidine (+0.76 V)
Neutral red (+0.24 V/-0.33 V)
Diphenylamine (+0.76 V)
References:
- ‘What Is a Redox Indicator in Chemistry?’ ThoughtCo, Available Here:
- ‘Redox Indicator’. Wikipedia, 8 Apr. 2022. Wikipedia, Available Here:
- Redox Indicator - Wikidoc, Available Here
- Sabnis, Ram W., et al. ‘Indicator Reagents’. Ullmann’s Encyclopedia of Industrial Chemistry, edited by Wiley-VCH Verlag GmbH & Co. KGaA, Wiley-VCH Verlag GmbH & Co. KGaA, 2009, p. a14_127.pub2. DOI.org (Crossref), Available Here:
Keywords:
Examples, internal, work, pharmaceutical analysis
You may also like this:
No comments:
Post a Comment