The major difference between suspension and solution is that the suspensions are a heterogeneous mixture, which means that the components do not mix completely and will most likely separate in the near future whereas, the solutions are homogeneous mixture since the components mix well together and remain mixed without separation.
Suspensions and solutions are both mixtures of various substances that are formed by combining one or more substances with different properties. Suspension, solution, colloids, and other dispersions are parallel however they have characteristics that differentiate each other.
| Table of Contents |
Suspension:
A suspension is a heterogeneous mixture of particles that have a diameter greater than 1000 nm and are visible to the naked eye. It is a heterogeneous mixture consisting of solid particles sufficiently large for sedimentation. All particles are completely mixed in this form of a mixture and can be seen under a microscope but light cannot pass through them.
When insoluble solid particles are dispersed in a liquid medium, they form a suspension. The solute particles are uniformly dispersed in the medium when shaken, but if left undisturbed, the solute particles, which vary in size from 0.5 to 5 μm, settle and can be isolated from the solution by filtration or centrifugation. They are classified according to their dispersion phase that can be solid and their dispersion medium which can be solid, liquid, or gas.
Examples of suspension:
- Sand in water
- Milk of magnesia
- Dusty air
- Paints in which dyes are suspended in turpentine oil
- Flour in water
- Muddy water
Solution:
A solution is a clear homogeneous mixture and solute particles no longer appear nor do they settle. The particles of solute have dimensions of molecular size and do not disperse the light beam passing through it. The solution has two components, solute, and solvent; a solute is a substance that is to be dissolved, and a solvent is a substance that dissolves the solute through a chemical change.
Since the composition is homogeneous, it is not possible to separate the solute from the solvent by filtering. Distillation is the only alternative, as it extracts the solvent while leaving a solid residue of the solute behind. The true solution, colloidal solution, and suspension are the three types of solutions that are classified based on the nature of particle sizes. These types of solutions show substantial differences in their nature, filterability, appearance, and color.
Examples of solution:
- Salt solution
- Alcohol in water
- Tincture of iodine
- Sugar solution
- Soda water
Difference between suspensions and solutions:
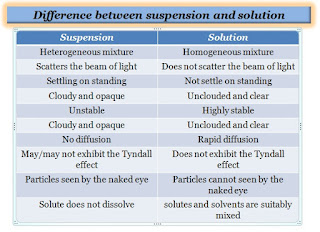
- Suspension is a heterogeneous mixture whereas the solution is a homogeneous mixture type.
- In suspension, the solute does not dissolve, it is suspended in the liquid and settled in the medium, while in solution, the solutes and solvents are suitably mixed in the liquid phase.
- Suspension scatters the beam of light, while the solution does not scatter the beam of light.
- The particle size of the suspension is more than 100 nm, whereas the particle size of the solution is less than 1 nm.
- Suspension may or may not exhibit the Tyndall effect which depends on the type of mixture, while the solution does not exhibit the Tyndall effect.
- The particles of a suspension can be seen by the naked eye whereas the particles of a solution are at the ion or molecular level and cannot be seen with the naked eye.
- The suspension is unstable, whereas the solution is highly stable.
- The suspension is cloudy and opaque, whereas the solution is unclouded and clear in appearance.
- The suspension is settling on standing, while the solution is not settled.
- There is no diffusion in the suspension, while rapid diffusion occurs in the solution.
- The suspension particle does not pass through the filter paper, while the solution passes through the filter paper.
Suspension VS Solution
Commonly asked questions are as follows.
What are the different types of the solution?
Solid-solid, solid-liquid, solid-gas, liquid-solid, liquid-liquid, liquid-gas, gas-solid, gas-liquid, and gas-gas are the types of solution.
How is a solution similar to a suspension?
Both solution and suspension are mixtures of two or more components and none of those components are chemically bonded together. The components in both solution and suspension can be isolated based on their physical properties by size, solubility, and density.
What are Colloids?
A colloid is a mixture in which one material is suspended in another by microscopically scattered insoluble particles. Example: milk, butter.
Which dosage form is semi-solid oil in water emulsion?
The cream is semi-solid oil in water emulsion that comes in two types: oil-in-water (O/W) and water-in-oil (W/O).
You may also like this
No comments:
Post a Comment