The major difference between paper chromatography and column chromatography is that the stationary phase in paper chromatography is cellulose paper, and it is based on the solid-liquid adsorption and solubility of the compound, whereas in column chromatography, a column packed with a matrix is used as a stationary phase to separate molecules. This separation is based on their size, affinity, or charge.
Chromatography is a significant biophysical method that allows the separation, purification, and detection of the analytes of complex mixtures for quantitative and qualitative analysis. Paper chromatography and column chromatography are both used in the separation of components in chromatography.
| Table of Contents: |
What is paper chromatography?
Paper chromatography is a type of planar chromatography method in which a piece of specialized paper is used as the stationary phase to separate the analytes. The principle involved is partition chromatography, in which substances are distributed or partitioned between liquid phases.
One phase is the water held in the pores of the cellulose filter paper used, and the other is the mobile phase, which moves on the paper. During the movement of the mobile phase, the compounds in the mixture separate due to variations in their affinity towards the stationary phase and mobile phase solvents. This mobile phase travels through the pores in the paper under capillary action.
What is column chromatography?
In analytical chemistry, column chromatography is a type of chromatographic technique for separating a chemical compound from a mixture that has been dissolved in a liquid. It separates sample molecules based on the adsorption of compounds, which allows them to be separated into fractions as the compounds pass through the column at different rates.
This process can be used to purify materials that will be employed on a smaller or larger scale in future research. This technique is an example of adsorption chromatography in which the stationary phase or adsorbent is solid. Silica gel is the most frequent stationary phase for column chromatography, followed by alumina.
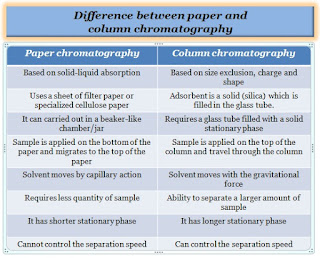
Difference between column and paper chromatography:
- Paper chromatography is based on solid-liquid absorption, whereas column chromatography is based on size exclusion, charge, and shape.
- Paper chromatography uses a sheet of filter paper or specialized cellulose paper as a stationary phase, whereas in column chromatography the stationary phase or adsorbent is a solid (Silica) which is filled into the glass tube.
- Paper chromatography can be carried out in a beaker-like chamber/jar, whereas column chromatography requires a glass tube filled with a solid stationary phase to perform the separation.
- In paper chromatography, a mixture of the sample is applied on the bottom of the paper and migrates to the top of the paper, whereas in column chromatography, the mixture of the sample is applied on the top of the column and travels through the column. For both techniques, the sample needs to be dissolved in a suitable solvent.
- The solvent in paper chromatography moves by capillary action, whereas the solvent in column chromatography moves with gravitational force.
- To perform the separation, paper chromatography requires less solvent, whereas column chromatography requires a larger amount of solvent system.
- Paper chromatography requires a smaller quantity of samples than column chromatography, which has the ability to separate a larger amount of samples.
- Since the stationary phase of paper chromatography is shorter, the sample is quickly separated, whereas column chromatography takes more time to separate the analyte as its stationary phase is longer.
- In paper chromatography, we cannot control the separation speed, whereas in column chromatography, we can control the separation speed by regulating the flow with a knob.
- Paper chromatography does not allow us to collect the sample, whereas column chromatography allows us to collect various bands into a separate container.
You may also like this
No comments:
Post a Comment