Mohr’s method is a type of precipitation titration that uses silver nitrate to determine chloride. For the argentometric determination of bromide, cyanide, and chloride ions, it employs the chromate anion (CrO42-) as the indicator.
Precipitation titration is a type of titration in which a precipitate is formed during the titration process. Precipitation titration refers to volumetric techniques based on the formation of a slightly soluble precipitate, while the argentometric method refers to titrations using silver nitrate (AgNO3) as a precipitating agent. In analytical chemistry, the most common use of this form of titration is to determine chloride using silver ions.
| Table of Contents: |
The titrant reacts with the solute to form an insoluble material in a precipitate titration. It uses silver ions to detect chloride levels and continues until all solutes are consumed. Depending on the type of application and the method of endpoint detection, precipitation titrations are classified as Mohr's method, Volhard method, and Fajan's method.
What is Mohr's method in chemistry?
The method of titration in which the chloride ion concentration of a particular solution is determined by titrating it with silver nitrate is called Mohr’s titration. Mohr's technique is still used in many laboratories and is one of the oldest titration procedures.
Precipitation titrations are a type of volumetric analysis that is based on the formation of compounds with limited solubility. Specifically, titration methods based on the use of silver nitrate as a precipitation reagent are called argentometric titration. Mohr's technique, named after German scientist and pharmacist Karl Friedrich Mohr (1806–1879), uses AgNO3 titration to estimate the quantity of chloride ions.
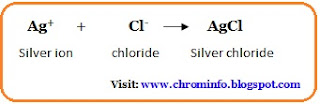
When all chloride ions interact with silver ions, the endpoint of the titration is observed using potassium chromate as an indicator, producing a brownish-reddish silver chromate precipitate. Silver chloride is precipitated during the titration Ag+ + C‑ - AgCl, and the appearance of silver chromate indicates the endpoint (reddish-brown precipitate) of the titration 2Ag+ + CrO42 – Ag2CrO4. When all chloride ions have reacted with the silver ions, the new equilibrium solid phase is formed.
Principle of Mohr's method:
The principle of precipitation titration is- Amount of added precipitating agent = the amount of a compound that is precipitated.
AgNO3 solution is used to titrate the chloride. As an indicator, a soluble chromate salt (K2CrO4) is used. This results in a yellow solution, when the chloride precipitation is complete.
NaCl + AgNo3 ⇆ AgCl + NaNo3
After all, chlorides have precipitated as silver chloride, the first excess of Ag+ reacts with the indicator to form red silver chromate as a second precipitate.
2 Ag+(aq) + CrO42–(aq) → Ag2CrO4(s)
Mohr’s method is a direct method of titration in which a red precipitate of silver chromate is formed at the endpoint. This method is needed to be performed in the neutral to alkaline condition (pH of 08.00).
Procedure of Mohr’s method:
Requirements: Burettes, conical flask, pipette, analytical/digital balance, 0.1 M silver nitrate solution, potassium chromate solution, and distilled water, etc.
- Fill the burette with a prepared solution of 0.05 N AgNO3.
- Pipette out 10.00 ml of sodium chloride solution (NaCl) and pour in a stoppered conical flask.
- Add 01.00 ml of 2% neutral potassium chromate indicator.
- By shaking the liquid continually, titrate it with 0.05 N silver nitrate solutions, until the red color formed by each drop of AgNO3 solution begins to fade more slowly; this indicates that the majority of the chloride has precipitated and the endpoint is approaching.
- Drop by drop; titrate until a faint but distinct, brick red color appears that does not disappear with vigorous shaking. The endpoint of this titration is a brick red color.
Detection of endpoint in Mohr’s method:
A small quantity of potassium or sodium chromate is added to the solution before titration to give it a slightly yellowish color. As long as chlorides are present during titration, the concentration of Ag+ is too low for silver chromate formation. Close to the equivalence point the concentration of silver cations increases rapidly, allowing precipitation of the intense red silver chromate, which indicates the endpoint of the titration.
Applications of Mohr’s method:
Mohr’s method is used to detect the concentration of chloride ions in water samples from a variety of sources, including river water, stream water, and a variety of pharmaceuticals and chemicals.
Advantages of Mohr’s method:
The Mohr technique has the advantage of being a direct, simple, rapid, and accurate technique for the determination of chlorides.
Frequently Asked Question (FAQ):
Which indicator is used in Mohr's method?
For the argentometric determination of chloride, bromide, and cyanide ions, the Mohr technique uses chromate ions (CrO42-) as the indicator.
Why Mohr's titration is performed in the neutral and slightly basic medium?
Since silver hydroxide forms at high pH while chromate forms H2CrO4 at low pH, the solution must be nearly neutral to reduce the concentration of chromate ions and delay the development of the precipitate.
Which type of solution is used in the Mohr method?
Mohr’s technique, which is used to estimate the chloride ion concentration of a solution by titration, uses silver nitrate solution.
What is the difference between Volhard and Fajan's method?
The Volhard method is an analytical technique used to determine the halide concentration using a back titration, while the Fajans method is an analytical technique by which we can determine the halide concentration by adsorption.
References:
- Wikipedia contributors. (2021, March 29). Argentometry. In Wikipedia, The Free Encyclopedia. Available Here:
- Mohr’s method challenge,Juris Meija, Available Here:
- Kotrly S, Sucha L. Handbook of Chemical Equilibria in Analytical Chemistry, Ellis Horwood Series in Analytical Chemistry. New York: John Wiley and Sons; 1985.
You may also like this
No comments:
Post a Comment